Gut bacteria: our friends or foes?
For many years bacteria or microbes have been associated with threats, diseases and fear, so unsurprisingly a lot of effort has been put into devising ways to eradicate them e.g antibiotics, disinfectants and hand sanitisers. However, in the last two decades, this approach has dramatically changed as research has shown that apart from pathogenic microbes, there are many types that help us thrive and we could not survive without them.
This piece focuses on the commensal gut bacteria and its role in health and wellbeing.
57% microbial, 43% human – what does it mean?
If we consider the type of cells we are made of, we are more microbes than human. Until recently, it was assumed that the number of bacteria outnumbered human cells by 10:1. However, this estimate has been recently revisited by researchers from the Weizmann Institute of Science, and now the most up-to-date estimate states that we are 43% human, with the ratio of human to bacterial cells closer to 1:11.
So how did researchers arrive at the 43% estimate? To calculate the number of bacterial cells in a human body, they used a “reference man”, who is between 20 and 30 years old, around 70 kg and 170 cm tall1. They calculated that his body would consist of about 39 trillion bacterial cells among 30 trillion human cells. So, by doing simple maths, 30x1012/(30+39)x1012 they arrived at 0.435 which equates to 43.5%.
We may be outnumbered by bacterial cells, but unsurprisingly our cells outweigh the bacterial ones, which weigh only approximately 0.2 kg across the whole human body.
Within the human body, microbes reach their highest density in the colon. Compared to the colon, the concentration of bacteria in the stomach and the upper 2/3 of the small intestine (duodenum and jejunum) is much lower. This is due to the relatively low pH of the stomach and the fast flow of the content through the stomach and the small intestine1. This may also explain why probiotic supplements have difficulty travelling through these environments.
The microbes inhabiting the gut are referred to as gut microbiota (microbial community, including bacteria, fungi, viruses etc.) or the gut microbiome (this term was originally used to refer to collective genomes harboured by microbes in the gut; however, nowadays ‘microbiome’ is colloquially used as a synonym of ‘microbiota’)2. They are a rich and complex community in which microbes interact with each other and with their host. These interactions have profound implications for human health and disease. Their importance for us becomes clearer when we look at the functions of the gut microbiome.
What does the gut microbiome do for us?
The gut microbiome can:
- break down food components which we cannot digest by ourselves (e.g. complex sugars e.g. oligosaccharides) [2].
- provide us with some vitamins (such B2, B6, B12, K, folate) which we cannot produce on our own [3].
- help us manufacture some neurotransmitters (e.g. serotonin)
- produce short-chain fatty acids (SCFAs) which can feed other beneficial bacteria, are a fuel for gut cells, and have a signalling function in the host’s body and organ systems
- assist in metabolism of bile acids
- reduce the risk of gut colonisation by pathogens (like Salmonella and E. coli)
- take part in development and modulation of the immune system[4].
- break down toxins and medications
In return, we provide them with nutrients from the food we consume, and a relatively stable environment to live in.
A few of the gut microbiome functions mentioned above are important for their interaction with the brain, known as the gut-brain axis. The gut microbiome plays an important role in mood, metabolism, cognition and motor function. Disruptions in the gut–brain axis have been associated with a number of conditions, including anxiety, depression, Multiple Sclerosis (MS), Parkinson’s Disease (PD), and autism5.
Gut microbiome
Scientists agree that the overall composition and function of the human gut microbiome is important for health. Although there is no checklist for ideal microbes, instead, diversity is understood as key.
A diverse gut microbiome means that there are numerous microbial species with different functionalities evenly distributed in the community, but at the same time, some of them are functionally related so that they could compensate for activities of species that may be lacking. In other words, it is a robust and resilient network of microbes which can take over each other’s work if necessary. Consequently, a diverse gut microbiome is seen as an indicator of a healthy gut2, [6].
It is therefore understood that lower bacterial diversity or impaired balance in the gut microbial community is less favourable for our health. It has been observed in people who suffer from irritable bowel syndrome (IBS), obesity, metabolic syndrome, inflammatory bowel disease (IBD), some types of cancer, type 1 diabetes, type 2 diabetes, atopic eczema, coeliac disease, psoriatic arthritis and arterial stiffness2. Recently, features of an aberrant gut microbiota has been also reported in autism, depression and Parkinson’s disease.
Symptoms associated with an altered gut microbiome include bloating, gas, diarrhoea, constipation, and weight gain (or difficulty losing weight).
What affects gut microbiome composition?
There is no such thing as a human gut microbiome signature species composition. Looking at the species level, in the gut of an adult there could be between 150 to 400 microbial species and the microbiome composition varies dramatically between individuals6, [7].
Although each person’s microbiome is unique, it changes over the course of their life and may still vary from day to day. In addition, some changes may occur following an infection, antibiotic treatment and significant changes in diet.
Despite prominent individual differences in microbiome composition, some common geographic and lifestyle patterns have also been identified. For instance, a Western-style diet (i.e. typical for the UK and the U.S.) contributes to the loss of 15-30% of gut microbial species as compared to those living in less industrialised areas. These differences have been attributed mainly to diet (high fibre and low sugar, fat, and meat in non-Western diets has been associated with bacterial richness in the gut), environment and access to healthcare (to medications, including antibiotics)8. In addition, westernisation of the gut microbiome has been recognised in recent immigrants from non-Western nations to the U.S. This demonstrates that diet, lifestyle and environment can induce profound and rapid changes to the gut microbiome. It is also an example of how resilient gut microbiota can suddenly be substantially altered.
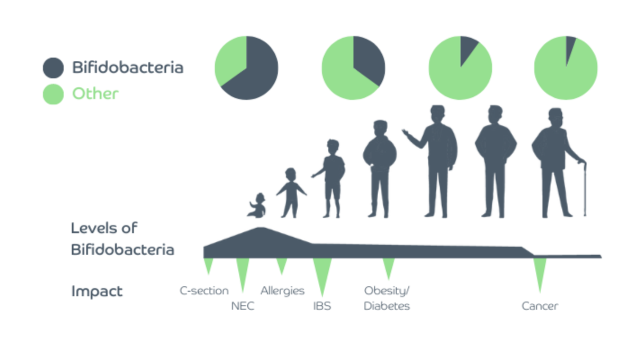
Microbes across lifespan
Another factor which affects the diversity and stability of the gut microbiome is age. Although during adulthood the gut microbiome is relatively stable, there are two periods in life when it undergoes a myriad of changes - early life and senescence[9].
Early life as an important beginning of gut colonisation
Our gut starts to get colonised at birth, with the bacteria resembling that of the mother’s vagina (if born vaginally), or that of her skin (if born by C-section). From birth to around 3 years of age gut microbiome diversity increases, and its composition starts to look like that of an adult. The process of establishing the gut microbiome is affected not only by the mode of delivery (vaginal vs C-section) but also gestational age, type of feeding (breast milk vs formula feeding), antibiotic usage, timing of the introduction of solid foods and cessation of milk feeding[10].
Early gut colonisation, between infancy and weaning, plays an important role in adequate development and modulation of the infant’s immune system, and thereby affecting protection against infections and the likelihood of allergy and/or atopy[11].
Studies show that in addition to reducing the risk of obesity, chronic diseases, such as diabetes and IBD, breastfeeding confers protection against respiratory, gastrointestinal tract infections and allergies. This indicates that diet significantly impacts infant health.
During the first year of life, human breast milk promotes a gut microbiome dominated by species of Bifidobacterium. Various components of human breast milk contribute to these effects, with Human Milk Oligosaccharides (HMO), which an infant cannot digest itself, having a prebiotic role by feeding beneficial microbes such as bifidobacteria, one of the most abundant gut bacteria in breast-fed infants.
Formula-fed infants have a different gut microbiome composition, with lower levels of Bifidobacterium compared to breast-fed infants[9].
Recently, researchers demonstrated that the gut microbiome of infants born by C-section and breast-fed, with time becomes comparable to those born vaginally[10].
Gut microbiome diversity decreases with age
Elderly people have a different gut microbiome profile compared to healthy younger adults. Generally, their gut microbiome diversity is decreased, and the overall composition is changed, with reduced levels of commensals such as bifidobacteria and lactobacilli, and increased levels of opportunistic bacteria[9].
These changes could be attributed to several features of ageing, such as changed lifestyle and dietary pattern, decreased mobility, reduced intestinal and overall functionality, recurrent infections, hospitalisations and use of medications. On the other hand, decreased gut microbiota diversity is associated with increased inflammation, which is a risk factor for many age-related diseases, including Alzheimer’s disease.
Given that gut microbiome diversity is currently perceived as one of key factors for healthy ageing, maintaining a healthy gut microbiome may lead to a reduced incidence of disease and contribute to longevity[9].
How to maintain a diverse gut microbiome?
A focus on diet
Diet emerges as one of the most important factors affecting the gut microbiome and at the same time appears as one of the ‘easiest’ to take control of, as simple steps can be taken to improve or maintain a healthy gut microbiome.
There are foods and diets proven to reduce microbiome diversity: artificial sweeteners such as sucralose, aspartame, and saccharin; emulsifiers such as polysorbate-80 and carboxymethyl cellulose; antiseptics such as potassium sorbate and sodium benzoate and restrictive diets such as gluten-free and low-FODMAP2.
Gut bacteria have their food preferences, and these foods produce predictable shifts in the existing gut bacterial community. Eating a varied, balanced diet is likely to lead to an equally diverse microbiome. It has been shown that a diet rich in prebiotics (fibre), polyphenols and omega-3 fatty acids is key for a healthy gut microbiome as they nourish the good gut bacteria[12].
References
- Sender et al.,PLoS Biology, 2016; 14(8):e1002533.
- Valdes et al., British Medical Journal, 2018; 361:k2179.
- LeBlanc et al., Current Opinion in Biotechnology, 2013; 24(2):160-8.
- Cani., Gut, 2018; 67:1716–1725.
- Knight et al., Annual Review of Genomics & Human Genetics, 2017; 18:65–86.
- Lloyd-Price et al., Genome Medicine, 2016; 8:51.
- Davenport et al., BMC Biology, 2017; 15:127.
- Vangay et al., Cell, 2018; 175, 962–972.
- Nagpal et al., The Journal of Nutrition, Health and Ageing, 2018; 4(4):267-285.
- Hill et al., Microbiome, 2017; 5:4.
- Tanaka et al., Allergology International, 2017; 66: 515-522.
- Lockyer et al., Nutrition Bulletin, 2019; 43:1-18.